Pharma Serialization

PHARMACEUTICAL SERIALIZATION AND TRACK & TRACE
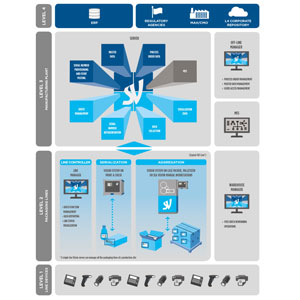
SEA Vision provides a complete solution to manage pharmaceutical serialization and track and trace. Thanks to a strong and configurable structure, this solution can safely satisfy the needs related to the ever-changing traceability regulations. The complete software suite covers from level 1 to level 3 and is composed namely: Site Server, HMI Supervisor, Vision systems for serialization and aggregation.
ADVANTAGES:
- Regulatory compliance: flexibility matches with performances.
Flexibility, scalability, and reliability are the main strengths of this serialization solution – not to mention complying with the new regulatory requirements that are coming into force throughout the globe. Thanks to the modular structure, which is built and designed on best practice, this solution can be tailored to each customer’s needs and can be upgraded at any time in order to comply with any future global pharma traceability regulations (e.g. DSCSA in the US, the Falsified Medicines Directive in Europe, CFDA in China).
- Hardware/software integration.
Our serialization solution has been developed with the highest integration capabilities to allow for easy connectivity with existing corporate systems (e.g. MES, ERP, Corporate Repositories or National Authorities). In addition, it provides the flexibility to adapt to both brand owner and CMO requirements in relation to serial numbers, data and event management process flows.
Providing our customers with a complete serialization solution that fully integrates into their packaging lines is one of our primary goals. Our R&D team has strong in the field experience with most OEMs, ensuring we design products that suit both existing and new machines.
- Maximize line OEE.
The strong integration with machines PLCs allows this solution to manage all the typical exceptions (machine jams, cartons/case rejections, etc.) occurring on the packaging line during the production process, thus ensuring safety whilst maximizing Overall Equipment Effectiveness (OEE). Following an advanced logic, through the exchange of I/O signals with the machine’s PLC, we can track objects and their status in every manufacturing phase. The efficiency of this integration approach results in reliable automatic anomalies management, and at the same time, it enables an accurate measurement of equipment performance.