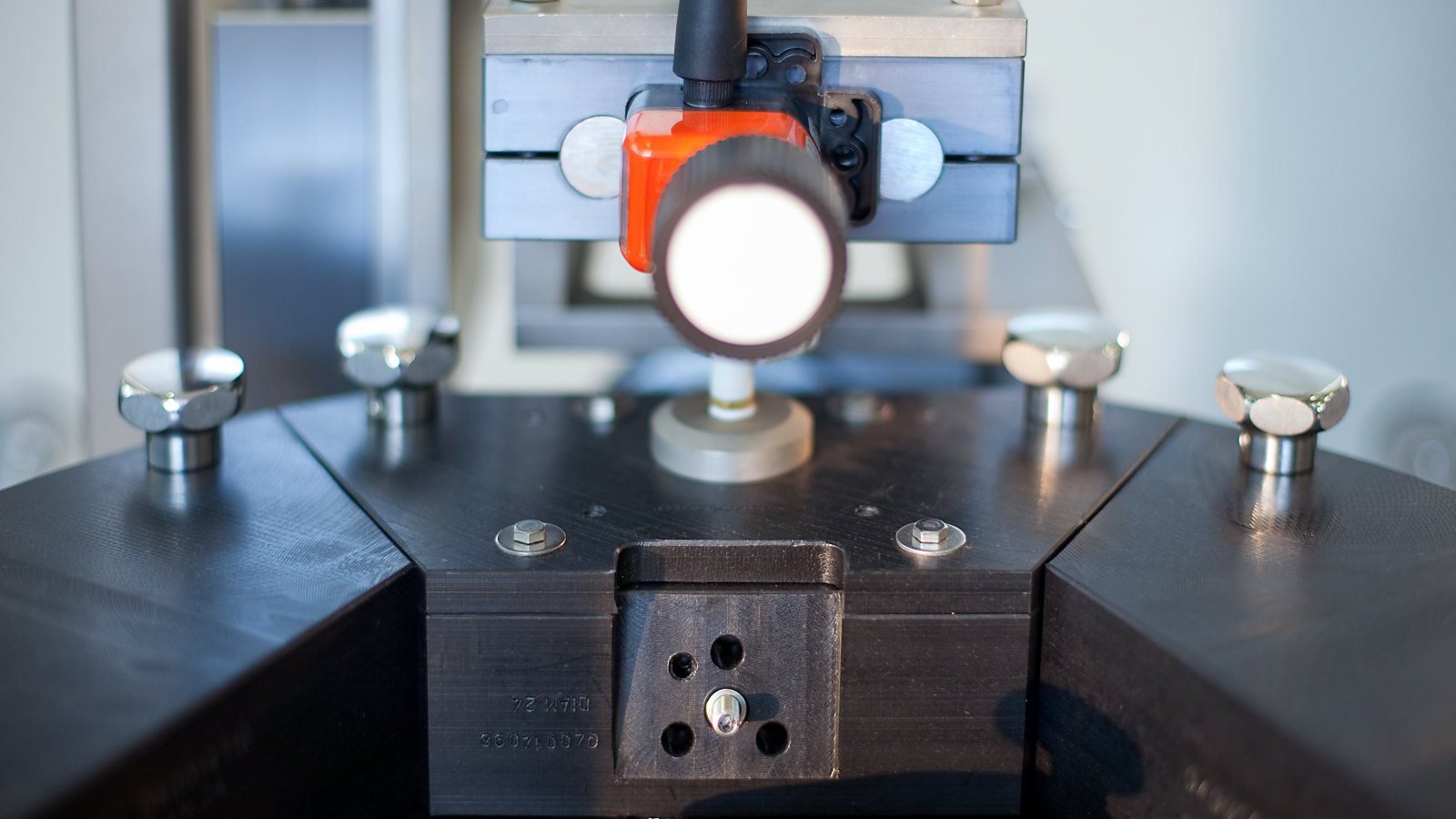
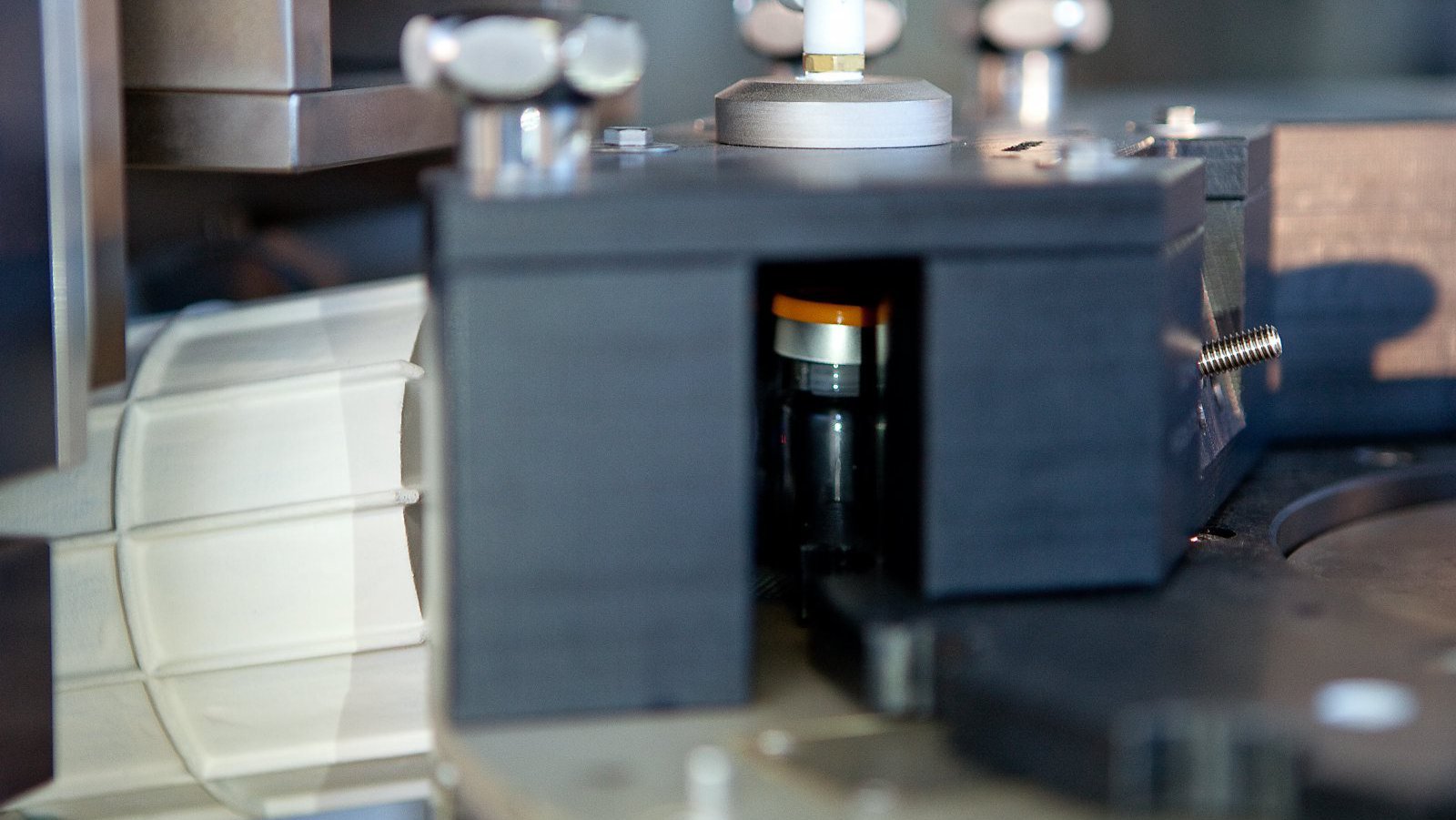
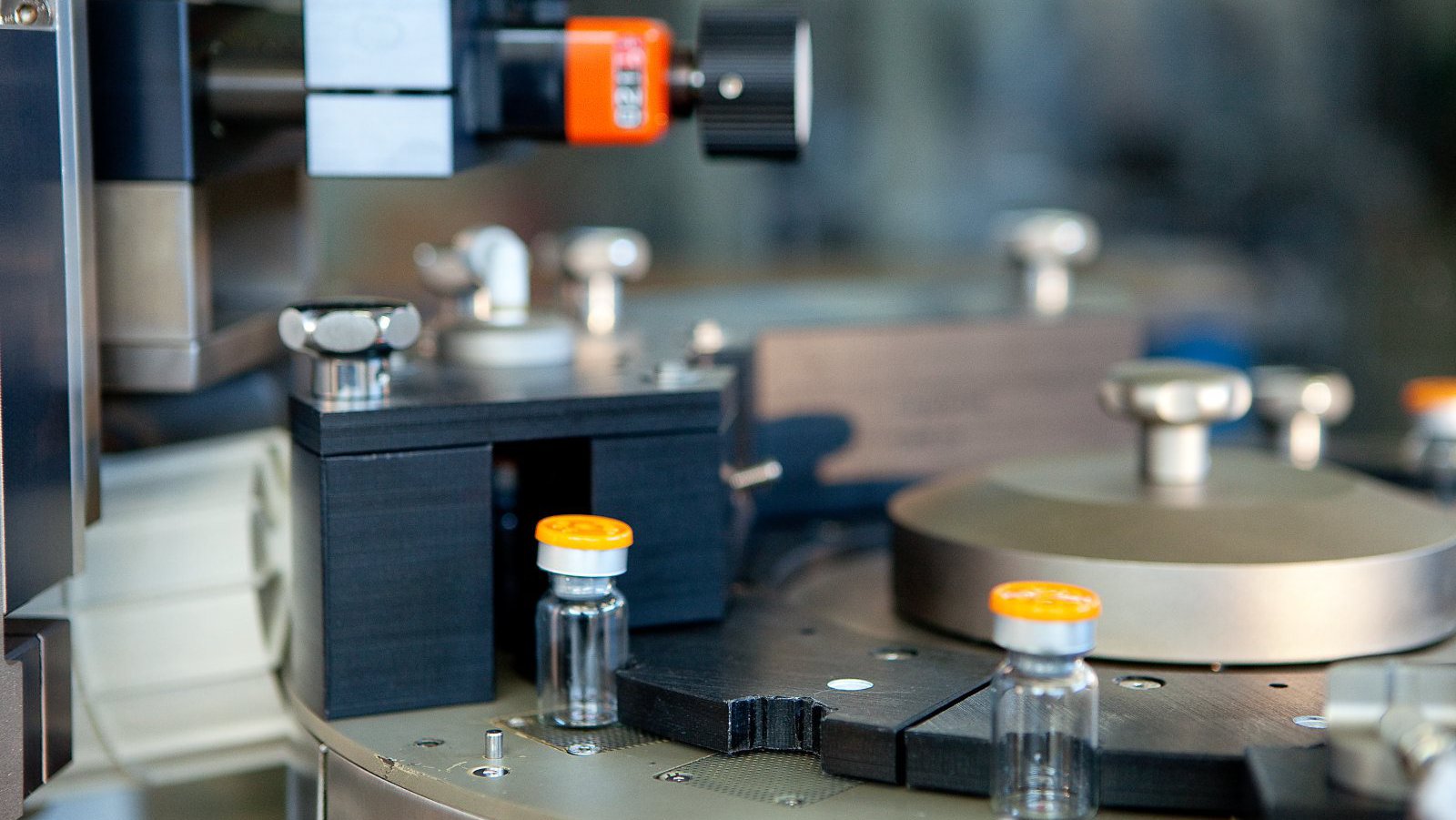
In-Line Headspace Gas Analyzer

Nitrogen purging not required No reference container required during operation Easily upgradeable High Versatility to achieve customizable solutions Automatic container rotation ensures highly accurate test results Extreme stability and accuracy even with limited headspace CFR part 11 compliance and 4.0 full integration
Technical Features
- Container Application:Vials, Ampoules, Cartridges, PFS
- Products: Powder, Lyo, Liquid
- Container Dimensions: Diam. 14 – 54 mm
- Fill Level: [1 – 100] ml
- Speed: up to 600 cpm
- Technology: HGA by Tunable Diode Laser Absorption Spectroscopy (TDLAS)
- Inspection Features: Non-intrusive, non-destructive, laser-based inspection method
- Inspection Capabilities: Oxygen/Moisture/Carbon Dioxide level, Absolute Pressure value
Additional Benefits
- Can be fitted with multiple laser heads depending upon production-line speeds
- Fast, reliable and repeatable results
- HMI real-time display of statistics and raw data
- Maximum accessibility of electrical and mechanical component for easy maintenance
- Connections to Active Directory and VPN router for machine remote access
- Computer-based automated measurement system: data acquisition, processing, and decision making.
- Validation package guarantees complete and efficient regulatory compliance
- Full batch control testing: fast, reliable and repeatable
- MES (Manufacturing Execution System connection) allows remote machine data exchange & download
Technology
- Headspace Gas Analysis (HGA) inspection process based on Tunable Diode Laser Absorption Spectroscopy (TDLAS) method to accurately quantify gaseous concentration levels. Diode laser beam transmitted through headspace region of the container and reflected back towards the receiver. LVA 600 sensors measure the laser beam absorption related to target gas concentration.
Quality Assurance
- Equipment test method refers to:
- EU GMP Annex 1 Manufacture of Sterile Medicinal Products, section 123: “Containers sealed under vacuum should be tested for maintenance of that vacuum after an appropriate, pre-determined period”
- Testing method in conformity with provisions of USP- United States Pharmacopeia – General Chapter <1207> “Package Integrity Evaluation – Sterile Products” (USP 39-NF34): Laser-Based Gas Analysis is listed among the Deterministic Leak Test Technologies
- Software designed to comply with FDA 21 CFR Part 11 and EU Annex 11
- Standard containers set supplied for easy calibration and verification