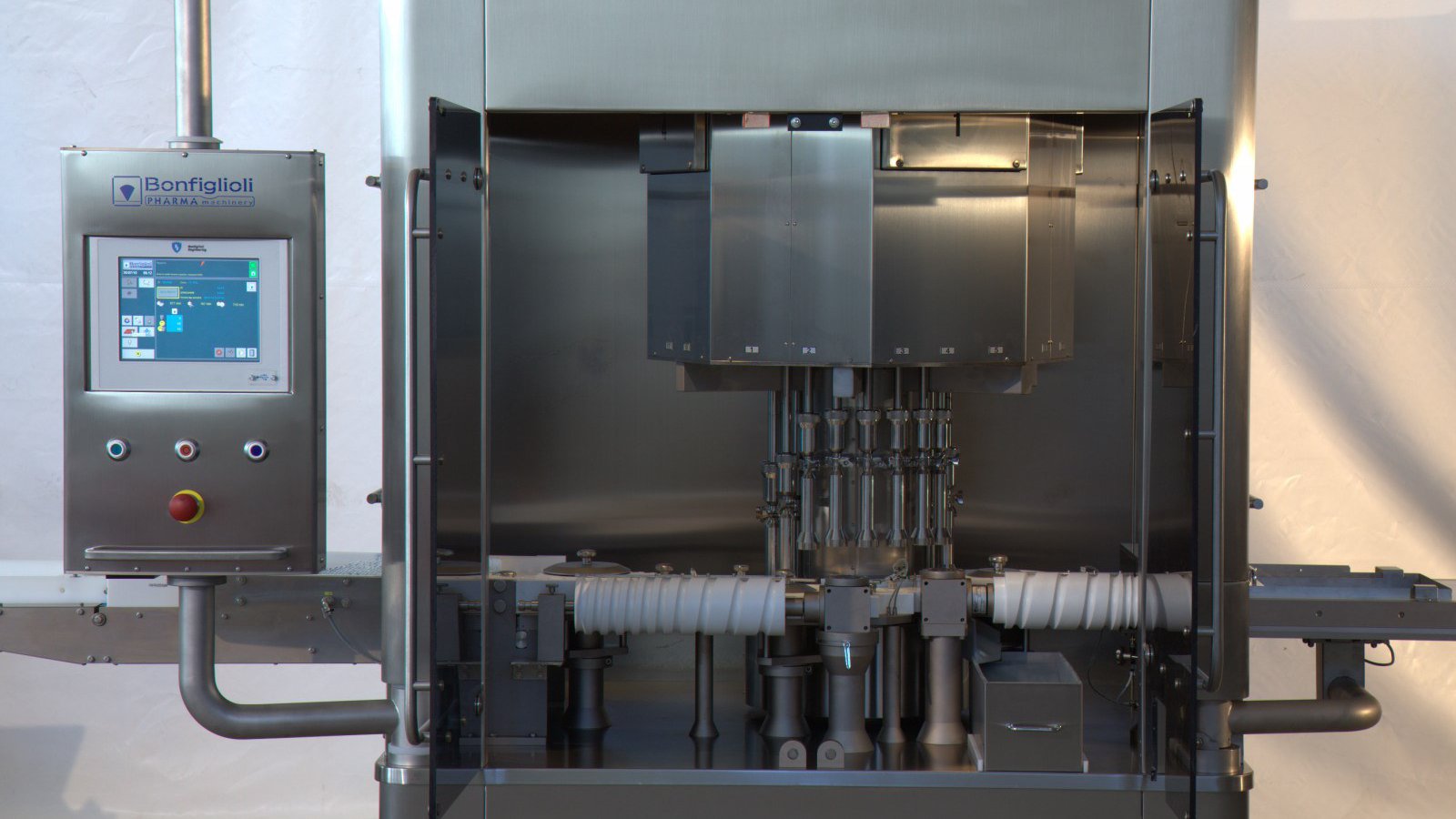
In-line Ampoules, Vials, Bottles and Cartridges CCI Tester

Autotest in real time Easy to validate CFR 21 part 11 compliance and 4.0 full integration Quick format changeover High installation flexibility Automatic Drying System, no testing chamber contamination (Vacuum Decay Only) Easy cleaning
Technical Features
- Container Application: Ampoules, Vials, Bottles (Glass + Plastic) and Cartridges
- Products: Solid, Semi-solid, Powder, Lyo, Liquid
- Container Dimensions: Standard Ampoules from 1 to 50 ml , Standard Vials and Bottles from 2 to 250 ml
- Speed: Up to 600 cpm
- Technology: CCIT
- Inspection Features: Non-Invasive, Non-Destructive CCIT based on Vacuum & Pressure Decay Method
- Inspection Capabilities: Microleaks detection; Tip Inspection integrity
Additional Benefits
- Full batch control testing: fast, reliable and repeatable
- Testing of all nominal production line speed
- MES (Manufacturing Execution System connection) allows remote machine data exchange & download
- Statistical Process Control reduces deviations for a better yield control
- Real time display of testing cycle diagrams, statistical raw data
- Easy, quick and safe remote access
- AHE (Automatic Head Exclusion)
Technology
- Container Closure Integrity Testing is a non-destructive measurement technology based on the following testing methods:
- Vacuum Decay Method
- Pressure Decay Method
- Measurement system comprises applying a pressure differential into an airtight testing group enclosing the container.
- The test objective is to detect container leakages by measuring the reached pressure level as well as the pressure change over test time.
Quality Assurance
- Equipment test method refers to:
- Approved industry standard “ASTM F2338-09”: “Standard Test Method for Non-Destructive Detection of Leaks in Packages”
- United States Pharmacopoeia – USP General Chapter «1207» “Packaging Integrity Evaluation”
- EU Guidelines to GMPMedicinal Products for Human and Veterinary Use –Annex 1 “Manufacture of Sterile Medicinal Products”
- PDA Technical Report No. 27 “Pharmaceutical Package Integrity”
- FDA 21 CFR part 11 as well as EMA Annex 11