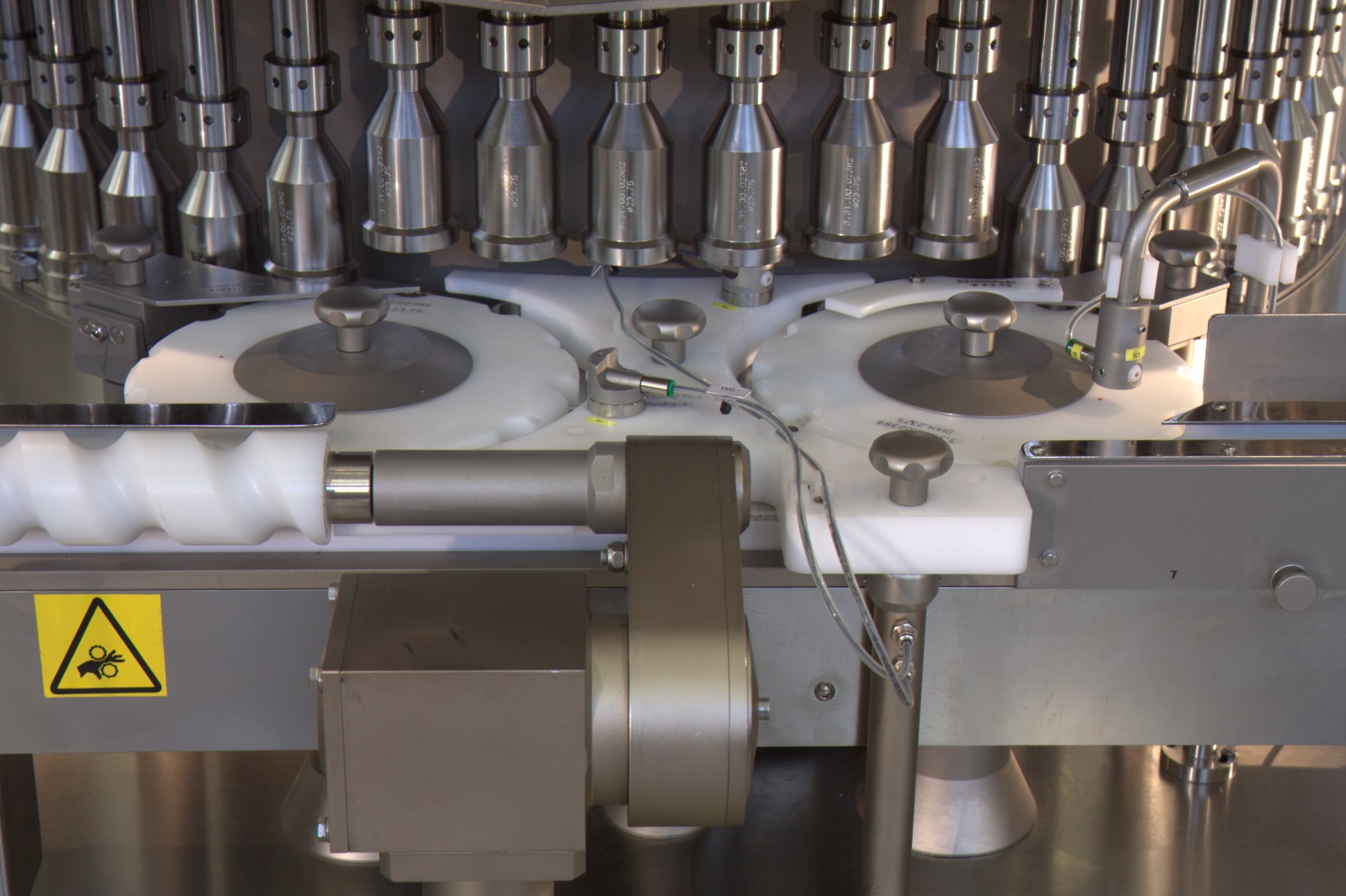
High Speed Vial Leak test machine

[popup title=”Click to open Video” padding=”20″ button=”1″][video_embed video=”” parameters=”” mp4=”http://www.acetechnologiesgroup.com/wp-content/uploads/2017/10/PK-V_short.mp4″ ogv=”” placeholder=”” html5_parameters=”” width=”700″ height=”400″] [/popup]
In-Line High Production Speed Leak Testing Machine
The PK-V Machine is designed to perform VDM based closure integrity testing of pharmaceutical vials and bottles by placing them in a vacuum test chamber.
It is fully suitable for both 100% in-line and off-line testing at high production speeds.
The leak test takes place into an airtight testing chamber in which a pressure differential is applied (Patent No. 1225063 of 13-9-1988). The test objective is to detect container leakages by measuring the reached vacuum level as well as the vacuum change over test time.
The leak testing machine measurement system is designed to identify the presence of leaks on containers due to:
- (micro) holes;
- Inappropriate sealing;
- Cracks.
Machine leak testing measurement system is fully conform to the approved industry standard ASTM F 2338-09 “Standard Test Method for Non-Destructive Detection of Leaks in Packages by Vacuum Decay Method”.
VDM technology is ideal for the closure integrity verification of liquid filled containers by measuring the loss of vacuum in the test chamber as a result of:
- Headspace gas leakage from the container, in case of leaks exposed to air / above the fill level.
- Volatilization of the container’s product located in or near the leak, in case of leaks exposed to product / below the fill level.
Container must be clean and dry.
A dual testing system is also available, allowing testing with both vacuum and pressure decay.
Powder, lyophilized and solid dose filled containers can be successfully tested by the PK-V machine.
Key benefits and differentiators of the TasiTest solution
Bonfiglioli Engineering was founded in 1974 expressly for the purpose of providing leak testing equipment for a wide range of products.
- The PK-V offers the following advantages for your application:
- Extensive pharmaceutical product testing experience goes into every PK-V machine.
- Bonfiglioli has delivered more than 2000 integrity machines of all types into the pharma market, with more than 200 systems for products very similar to those in your request. We fully understand the regulations, the construction techniques, material selection and validation of your market.
- We have been involved in the container closure integrity market since the ‘80s. We work closely with the manufacturers of the base technologies to meet the needs of the pharma test and inspection market and strive to stay current with emerging technology trends, incorporating improvements as newer technology stabilizes and becomes production ready.
- Bonfiglioli Engineering has conducted studies comparing the statistically significant differentiation capability of our system against others in the market and we are confident that our results are industry leading.
- Market leading test cycle time reduces overall time for batch testing.
- Barometric compensation system: it avoids any vacuum level readings variations due to environmental atmospheric pressure fluctuations.
- Convertible method of testing (vacuum VS positive pressure) with no need to change mechanical components.
- We have used our experience in pharma market and other regulated industrial sectors to develop a highly functional, intuitive HMI and control system that is robust, easy to use, displays real time testing cycle diagrams, statistical and raw data.
- Bonfiglioli’s VDM systems offer several best in class features all combined into one unit. Some of these features are:
- Containers automated loading and unloading;
- High leak detection sensitivity;
- Fast, reliable and repeatable test results;
- Non-invasive and non-destructive test method;
- System auto diagnostics available;
- Low maintenance;
- Easy to clean – no hidden corners;
- Ease of maintenance – free access to all moving parts and adjustment points;
- Cost-effective solution;
- Quick change over for all format specific tooling;
- Storage, maintenance, and download of historical data (production, tests, events and alarms);
- HMI real time display of leak testing cycle diagram;
- Validation package guarantees complete and efficient regulatory compliance.
- Extensive pharmaceutical product testing experience goes into every PK-V machine.